

As the first and only riluzole formulation that dissolves on the top of the tongue,

RILUZOLE PILL

Not actual size. Size and shape of
A different way to deliver riluzole
For patients who have difficulty swallowing some medications, EXSERVAN™ may be an option.1-3
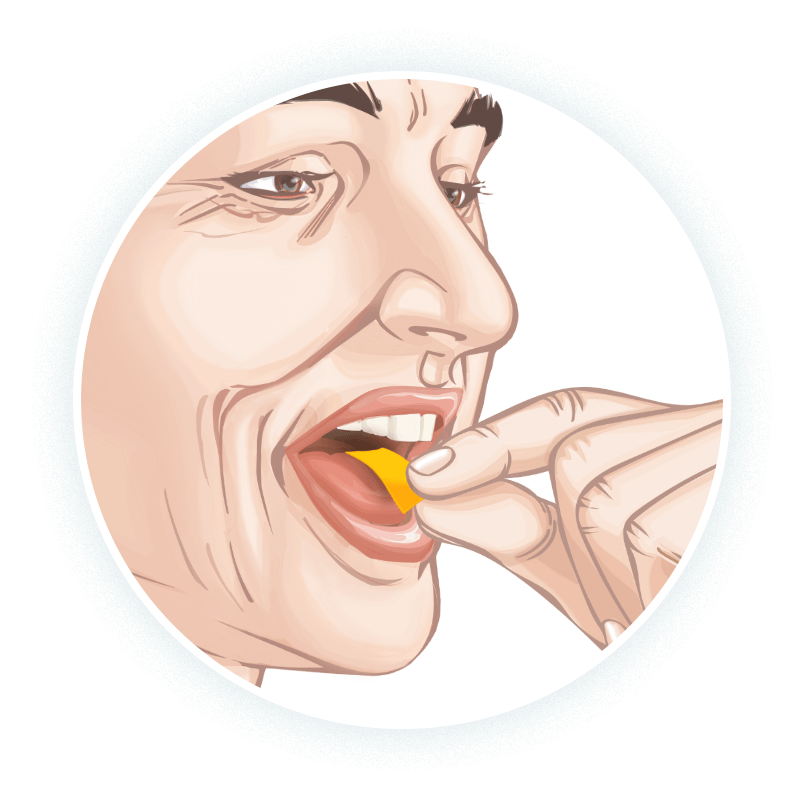
- The first and only oral film formulation of riluzole1
- Dissolves on the top of the tongue to deliver 50 mg
of riluzole1 - Demonstrated bioequivalence to riluzole tablets (RILUTEK®) with a similar rate of absorption1,3,5
- Safety and tolerability similar to riluzole tablets1,5
Committed to delivering informative resources to physicians and their patients
Mitsubishi Tanabe Pharma America, Inc. is committed to advancing treatment options for people living with ALS.
Discover another treatment option to consider in your patients' ALS journeys
Learn MoreLearn more about the first and only
-
References:
EXSERVAN™ (riluzole) Prescribing Information. Jersey City, NJ: Mitsubishi Tanabe Pharma America, Inc.; 2021.- Data on File. Mitsubishi Tanabe Pharma America, Inc., Jersey City, NJ.
- Center for Drug Evaluation and Research (US). 2017. EXSERVAN (riluzole). Washington DC: US Food and Drug Administration, Center for Drug and Evaluation Research. https://www.accessdata.fda.gov/
drugsatfda_docs/ nda/ 2019/ 212640Orig1s000MedR.pdf. Accessed April 2, 2021. - Tomik J, Sowula K, Dworak M, et al. Esophageal peristalsis disorders in ALS patients with dysphagia. Brain Sci. 2020;10(11):820.
- RILUTEK® (riluzole) Prescribing Information. Cary, NC: Covis Pharmaceuticals Inc.; 2016.
- National Institute of Neurological Disorders and Stroke. Amyotrophic Lateral Sclerosis (ALS) Fact Sheet. https://www.ninds.nih.gov/
disorders/ patient-caregiver-education/ fact-sheets/ amyotrophic-lateral-sclerosis-als-fact-sheet. Accessed April 15, 2021.